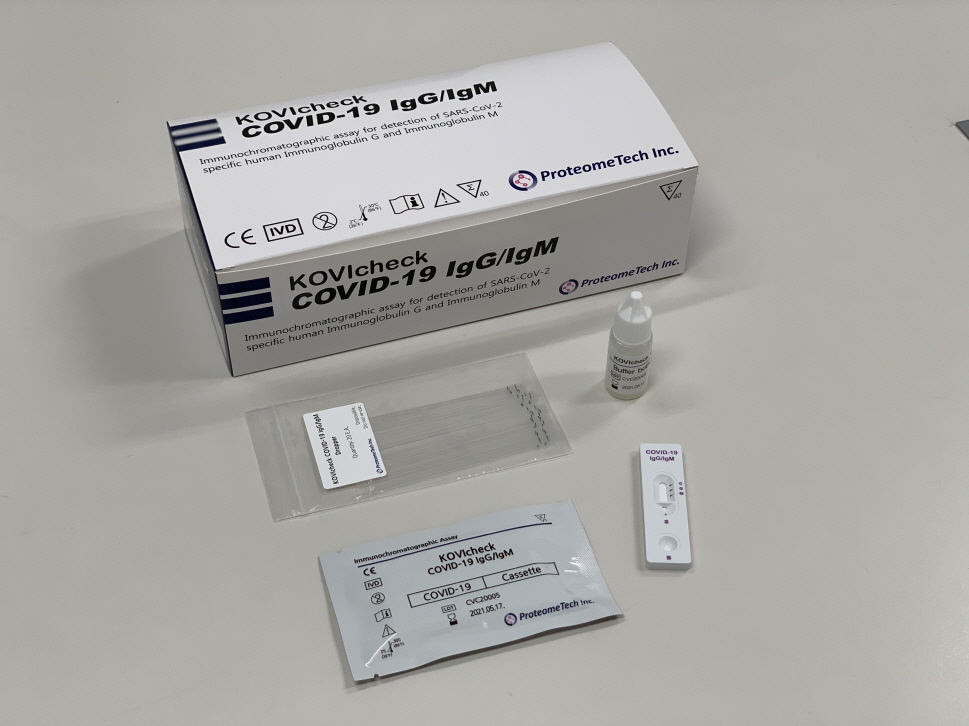
GENEdania COVID-19 qRT-PCR (ProteomeTech Inc)
GENEdania COVID-19 qRT-PCR
- HS Code
- Certification CE-IVD, FDA EUA Notification
- Patent
- Awards
- Features
The GENEdania COVID-19 qRT-PCR is an in vitro diagnostic (IVD) one step real-time RT-PCR kit for the detection of nucleic acid from SARS-CoV-2. It targets the Orf1ab gene and the N gene for SARS-CoV-2 (COVID-19), specifically. The E gene for beta coronavirus and endogenous control gene for kit verification are also included in a single tube. All four genes are amplified simultaneously in one tube and give the accurate results less than 2 hours from purified COVID-19 RNA samples. This kit can be applied to the sample from upper respiratory specimens e.g. nasopharyngeal, oropharyngeal swab and lower respiratory specimens, e.g. sputum, from individuals with signs and symptoms of COVID-19.
Company Information
Address : A-702 Gangseo hangang Xi Tower, 401 Yangcheon-ro, Gangseo-gu, Seoul 07528 Korea
Website : http://http://www.proteometech.com/
Proteometech Inc. aims to contribute to human health and well-being through finding new protein targets for disease prediction, early diagnosis, and development of new medicines. Based on our dedication for identification of bio-markers needed in early diagnosis of incurable diseases with and our advanced technology in "protein analysis" we have successfully developed unique in vitro diagnosis merchandise such as allergy diagnosis kits, immunity monitoring kits and next-generation pregnancy diagnosis kits, and are currently developing early cancer diagnosis kits. As a manufacturer of award-winning in vitro diagnostic medical devices (Jang Yeongsil Award, New Excellent Technology (NET), World-Class Products and more), Proteometech is a group of talented individuals striving for production of the World's Best Products with strong passion and skills.