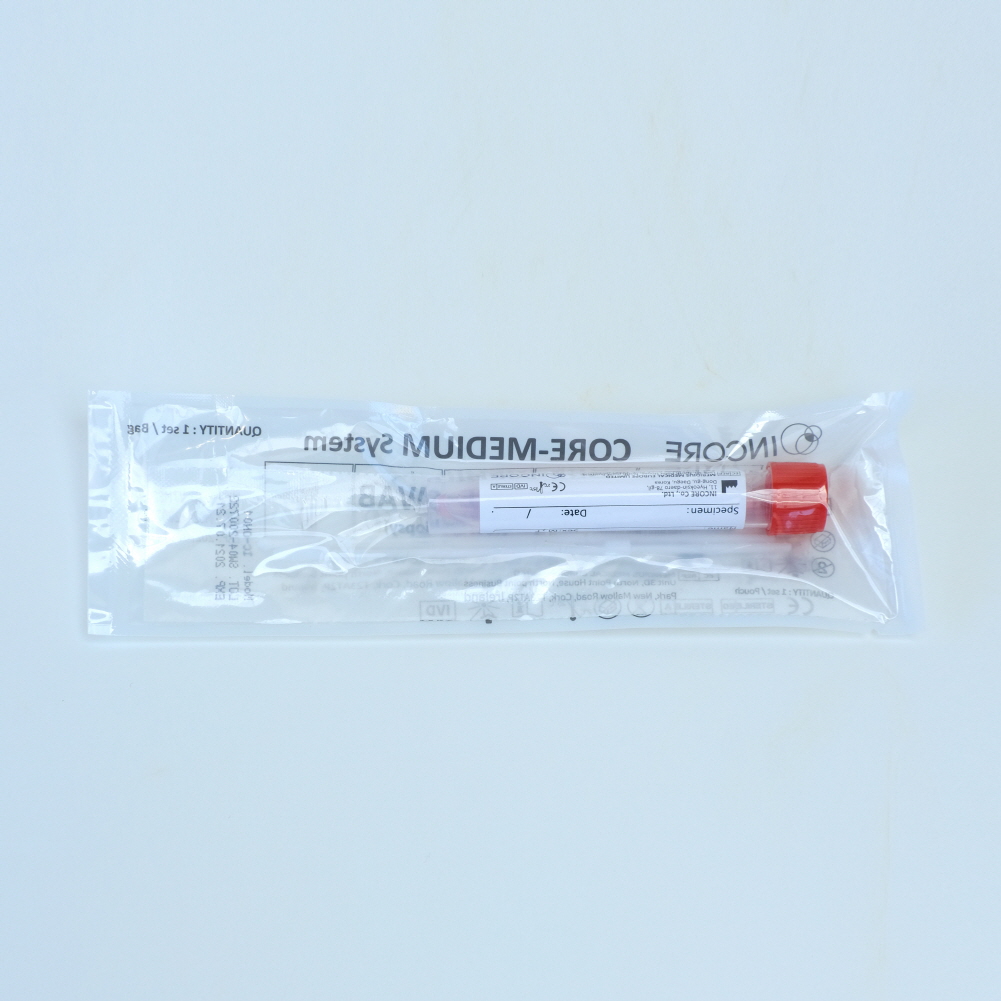
CORE-SWAB (INCORE Co., Ltd.)
CORE-SWAB
- HS Code 6307.90.9000
- Certification Permitted by KFDA, Registered on FDA
- Patent
- Awards
- Features
CORE-SWAB is made of thin plastic which prevents contamination of the specimen.
Its ergonomic design minimizes the uncomfortableness during the collection.
* Permitted by KFDA, Registered on FDA
Product details
CORE-SWAB is basically compose of two swabs: one is a nasal swab and the other is a oral swab.
Swabs are ergonomically designed to enhance the usability as well as to decrease the discomfort of people undergoing the tests.
The body of the nasal swab is thin and flexible. it is more acceptable visually and sensually.
The swab is flexible, however, it is durable enough to prevent swab-breaking accident. It is designed to be broken at the break point by only the users' intention.
Company Information
Address : 11, Hyeoksin-daero 78-gil, Dong-gu, Daegu, Republic of Korea
Website : http://www.incoremedi.com
We, INCORE Co., Ltd, are a medical consumables manufacturer with the top notch technology, distributing our products over the world.
Fully aware that in the 21centuary high quality of a product at a competitive price meets consumers' demand, we are growing to contribute to the society with young human resources and swift market reaction.
Under the mission of customer satisfaction, we do our best to meet the delivery date and demand for high product quality with stringent management over the associates and sophisticating system.
Fully aware that in the 21centuary high quality of a product at a competitive price meets consumers' demand, we are growing to contribute to the society with young human resources and swift market reaction.
Under the mission of customer satisfaction, we do our best to meet the delivery date and demand for high product quality with stringent management over the associates and sophisticating system.