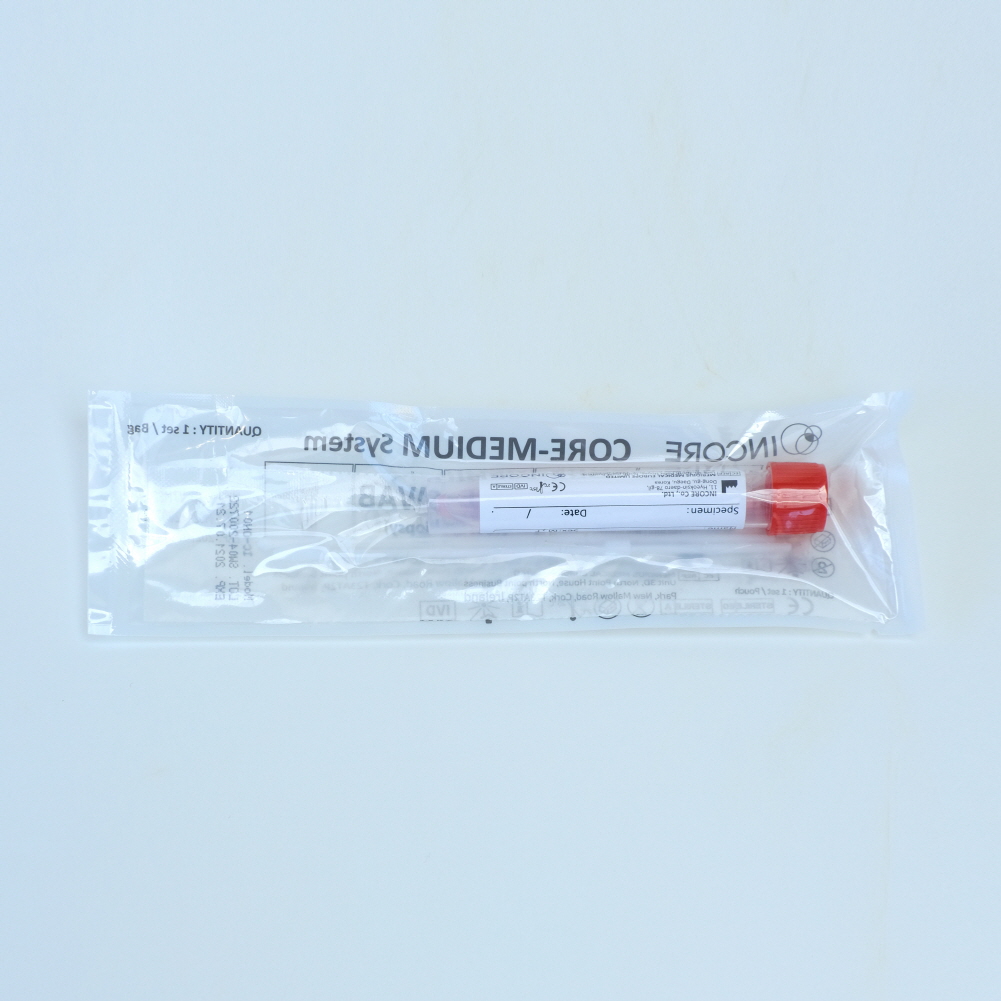
CORE-MEDIUM System은 (INCORE Co., Ltd.)
CORE-MEDIUM System은
- HS Code 3821.00.0000
- Certification Permitted by KFDA, Registered on FDA, Holding CE DoC, Registered on HPRA Ireland, Approved by NHS UK
- Patent
- Awards
- Features
Clean, Safe collection kit made in South Korea.
CORE-MEDIUM System consists of a specimen collecting swab(s) and a tube that contains Universal / viral transport media. The product is designed to collect specimens safely and to ensure accurate detection of the disease.
We believe the accuracy of the detection test can enhance the quarantine effort of all the social members. Therefore, we focused on the quality of each component of CORE-MEDIUM System. Also, we test our products with Laboratory Animal Resource Center in Daegu Gyeongbuk Medical Innovation Foundation to check and to ensure the quality.
Our products are made in a controlled environment. Furthermore, skilled workers produce the products safely
* Permitted by KFDA, Registered on FDA, , Holding CE DoC, Registered on HPRA Ireland, Approved by NHS England.
Product details
CORE-SWAB and CORE-MEDIUM are the components of CORE-MEDIUM System. The product is specimen collection kit.
CORE-SWAB is basically composed of two swabs: one is a nasal swab and the other is a oral swab.
Swabs are ergonomically designed to enhance the usability as well as to decrease the discomfort of people undergoing the tests.
The body of the nasal swab is thin and flexible. it is more acceptable visually and sensually.
The swab is flexible, however, it is durable enough to prevent swab-breaking accident. It is designed to be broken at the break point by only the users' intention.
CORE-MEDIUM is Universal / viral transport media to transport specimen safely to the labs or institution with diagnostic devices.
CORE-MEDIUM System is sterilized product. Do not use if the package of CORE-SWAB or CORE-MEDIUM is damaged.
Please, use the products with caution. Unintentionally contaminated kit and specimen could lead to wrong diagnostic results.
The how-to-use is as follows.
1) Open the package of the products
2) Collect the specimen with caution
3) Put the swab head in the vtm tube and break it at the break-point
4) Close the lid completely
5) Fill out the form on the tube with the correct information
6) Transport to the diagnostic agencies as soon as possible
Company Information
Address : 11, Hyeoksin-daero 78-gil, Dong-gu, Daegu, Republic of Korea
Website : http://www.incoremedi.com
Fully aware that in the 21centuary high quality of a product at a competitive price meets consumers' demand, we are growing to contribute to the society with young human resources and swift market reaction.
Under the mission of customer satisfaction, we do our best to meet the delivery date and demand for high product quality with stringent management over the associates and sophisticating system.